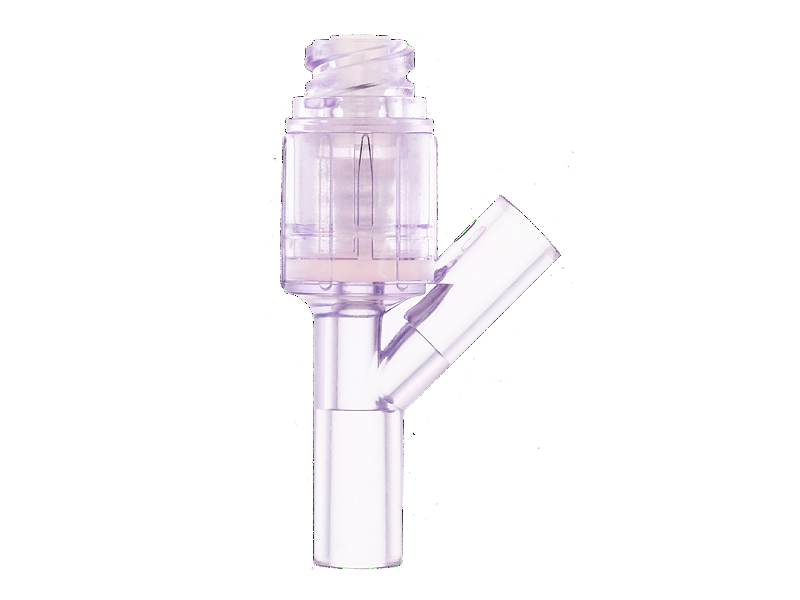
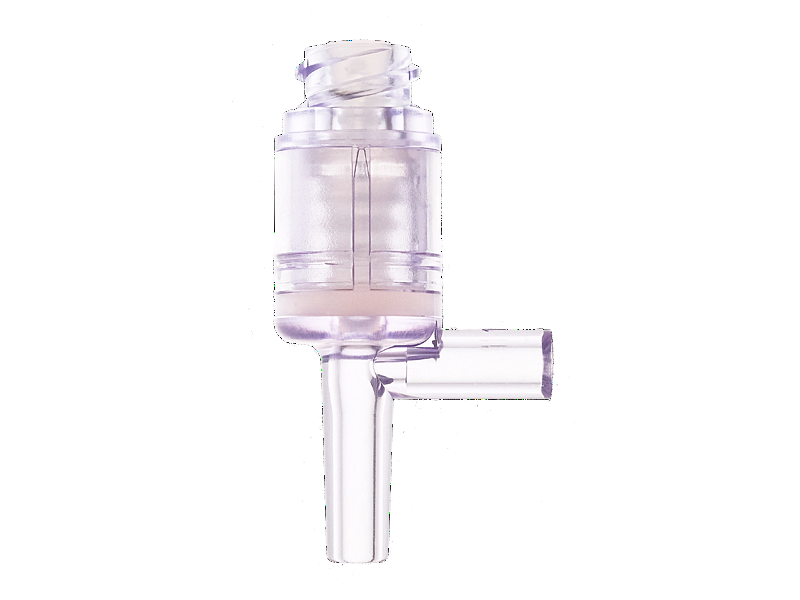
Needleless infusion devices develop and produce by Chifeng plastic, and acquired patents from multiple countries. Compared with Gen 1, the Gen 2 needleless infusion series using the neutral pressure design, minimized the pressure from insert and pull out the needle, in order to reduce the pain of discomfort of the pressure changing from a patient.
Metal components are not included in this product, in order to avoid components malfunction or affect the MRI. The fitting part follow the ISO 594 standards and complete the test, can fit any infusion products who follow the ISO 594 standards. Housing and septum material is using medical grade polycarbonate and medical grade silicone, easy to visualize the fluid path during operation, bio-caompatibility test pass, lipid and alcohol resistance. Silicone seal can activation up to 200 actuations, and the swabable surface is sealed arround the top of housing, to create first microbial barrier protection. In the fluorescein dye test.
According to the fluorescein dye test, when disconnect and swab the surface, the fluorescein dye did not residual on the swabable surface. When need to using the Gen 2 needleless series in the high pressure infusion therapy, it has #16C002 to provide high pressure infusion therapy, it can withstand the pressure from pipeline up to 22.8kg/cm2 (325PSI).
TO SEE #Superior Chemo drug preparation system #18C025
 Comply with the ISO 594 standards, and can comply with most other infusion products.
Comply with the ISO 594 standards, and can comply with most other infusion products. The swabable surface design fits most clinical practices, easy to swabbed and no residual on the surface.
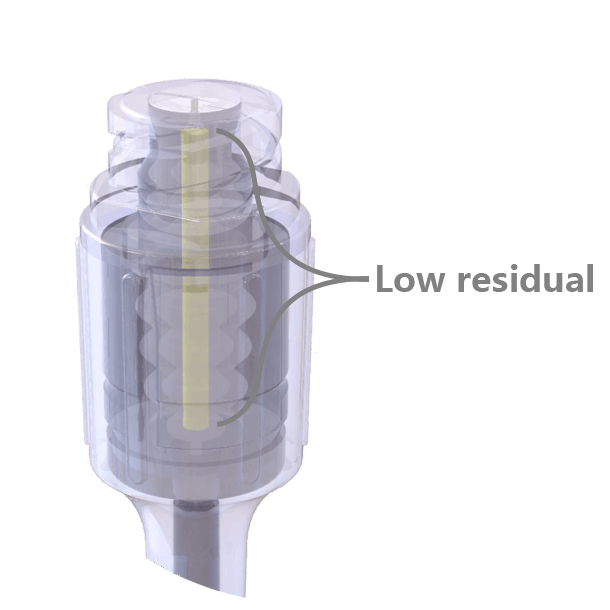
The swabable surface design fits most clinical practices, easy to swabbed and no residual on the surface.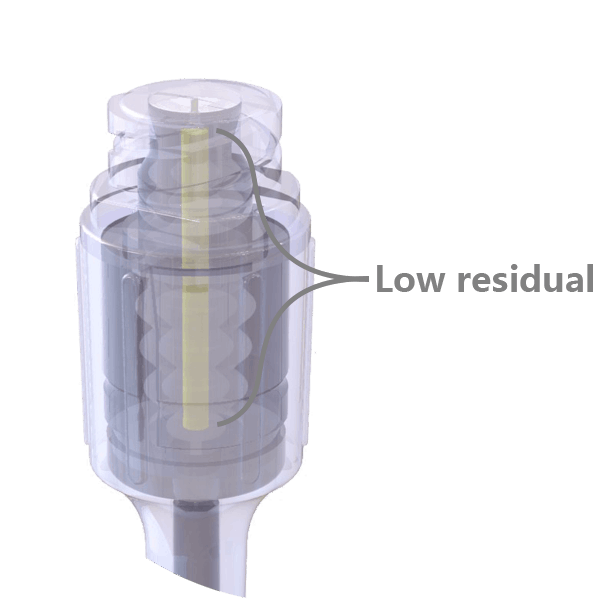 Neutral pressure and low residual design, only 0.016ml priming volume.
Neutral pressure and low residual design, only 0.016ml priming volume. Transparent appearance, easy to visualize the fluid path and to operate.
Transparent appearance, easy to visualize the fluid path and to operate. Design of miniaturization, suitable for pediatrics or other small needleless infusion device.
Design of miniaturization, suitable for pediatrics or other small needleless infusion device.